News this week that new blood test technology can reliably detect Alzheimer’s disease was met with excitement – but a dementia specialist is warning that the accuracy of these tests varies.
“Some of the blood tests are accurate and some are not, and doctors don’t know which tests to use,” explained Dr. Suzanne Schindler, an associate professor of neurology at Washington University in St. Louis. “With this head-to-head comparison, doctors now have more reliable information about which tests will best help them provide an accurate diagnosis for their patients.”
Schindler led a study that compared the accuracy of six commercial blood tests—four of which are clinically available.
Historically, Diagnosing Alzheimer’s, the most common type of dementia, which affects more than 5.8 million Americans, required invasive and expensive screening methods, such as spinal tap or brain scan.
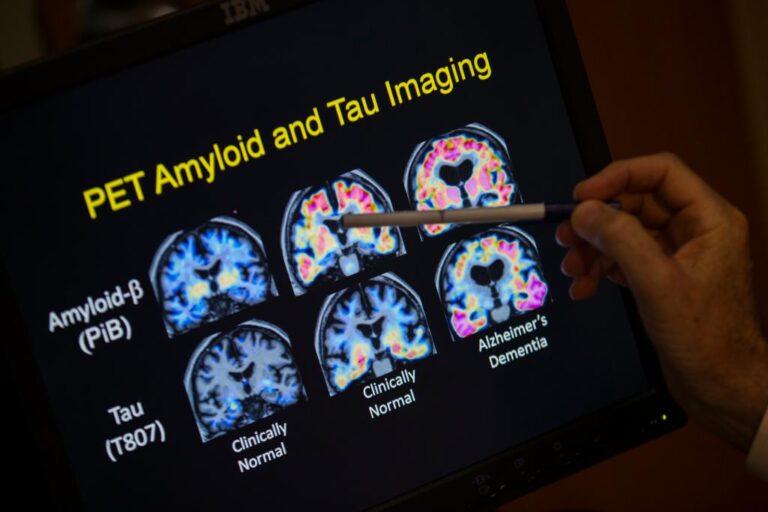
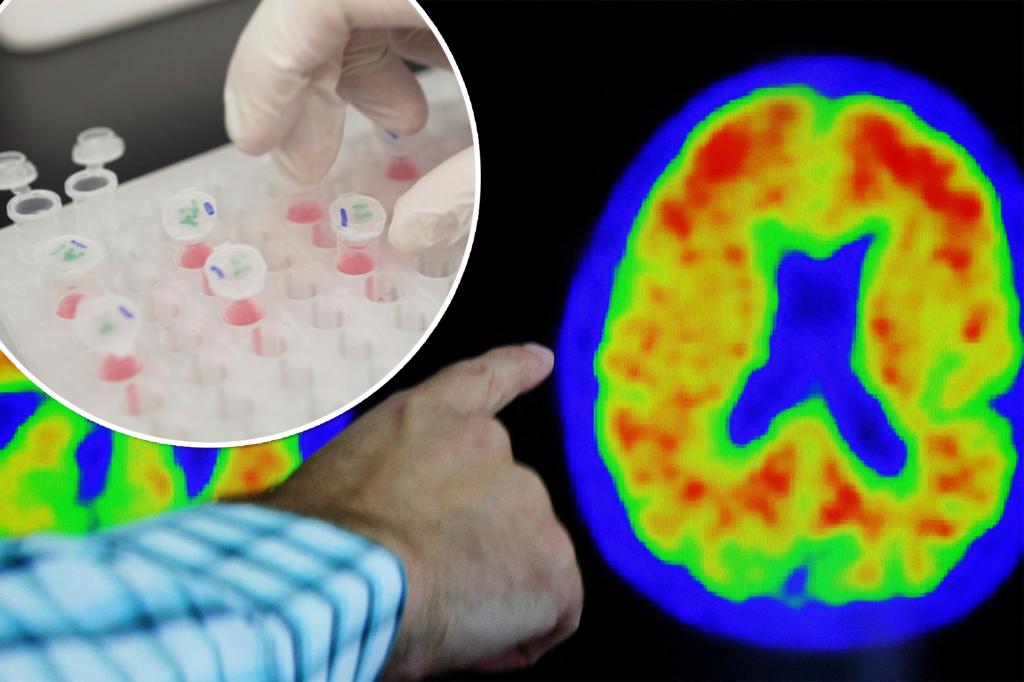
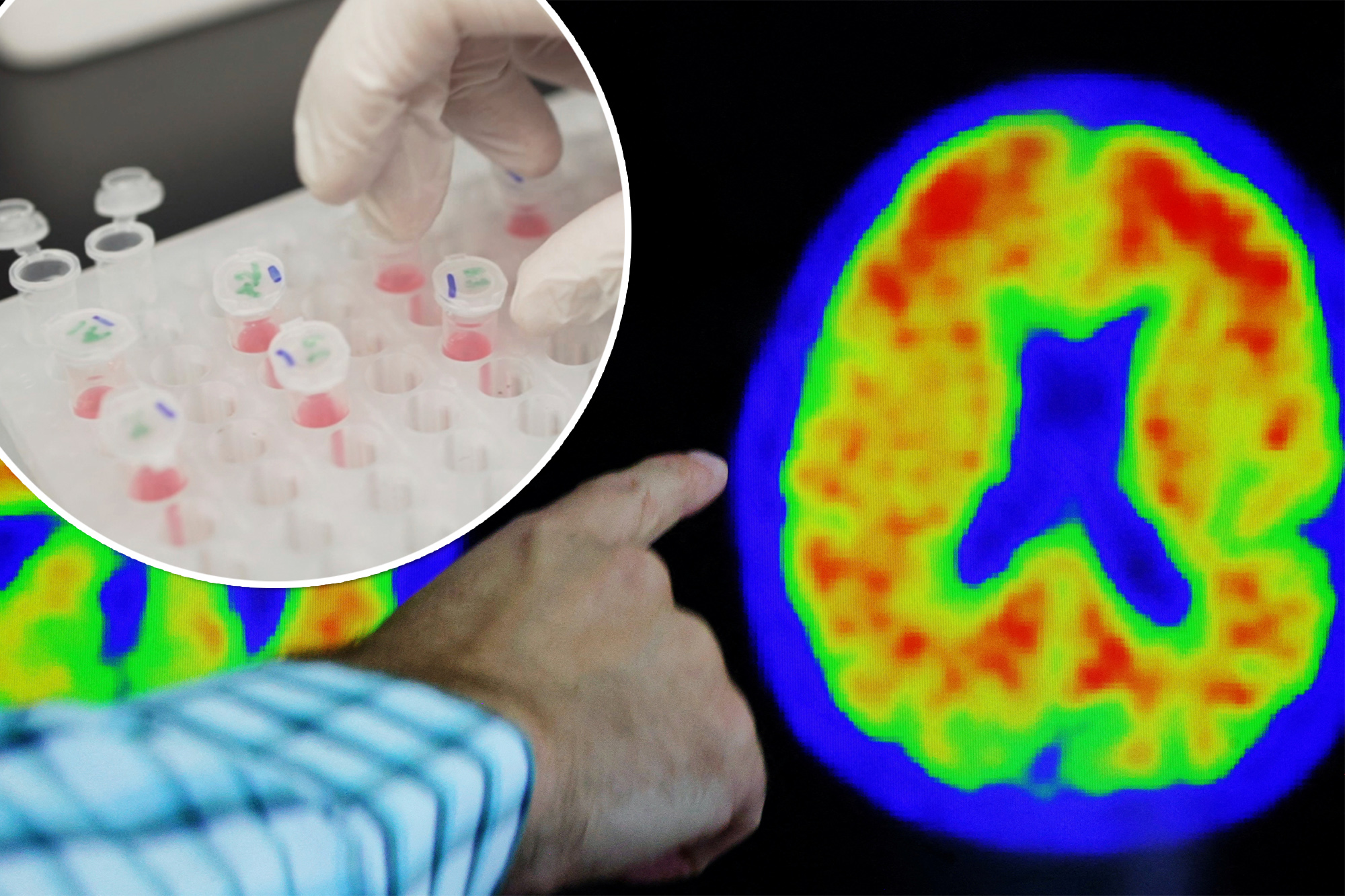
Telltale biomarkers of the disease are clumps of brain-clogging proteins called amyloid plaques and the accumulation of abnormal tau protein.
Schindler’s study included 392 people who gave blood samples within six months of a brain scan. The participants were mostly around 80 years old – almost half of them showed cognitive problems.
Each of the six tests measured blood levels of one or more Alzheimer’s biomarkers, such as amyloid plaques, abnormal tau protein, reduced brain volumes and cognitive impairment.
One biomarker, used in four of the tests, was remarkably accurate in identifying signs of Alzheimer’s – a form of tau known as phosphorylated tau 217 (p-tau217).
“Some people thought we might need to measure multiple biomarkers to understand the different features of Alzheimer’s disease,” said Kellen Petersen, an instructor in neurology at the WashU School of Medicine.
“That’s not what we found,” Petersen continued. “Only P-tau217 can do it all. It accurately predicted levels of amyloid and tau in the brain, brain volumes and cognitive symptoms. It was more accurate than any other biomarker, or even any combination of biomarkers, across the board.”
The four p-tau217 blood tests performed well regardless of how the biomarker was measured.
“We concluded that, to be used without a second test, blood tests must be as accurate as [FDA]-Approved cerebrospinal fluid tests that are approximately 90% sensitive and specific in identifying Alzheimer’s disease in cognitively impaired individuals, Schindler said. “In this current study, the p-tau217 tests met that standard, but the others did not.
Petersen presented the research Tuesday at the Alzheimer’s Association International Conference in Philadelphia.
The FDA has approved only two Alzheimer’s drugs, with more in the pipeline.
Leqembi and Kisunla have been shown to modestly slow the worsening of symptoms by targeting and removing amyloid plaques in the brain.
Both drugs work only in the earliest stages of the disease, making early detection, such as that promised by these blood tests, essential for effective treatment.
By postal wire
#Accuracy #blood #tests #Alzheimers #disease #varies #study
Image Source : nypost.com